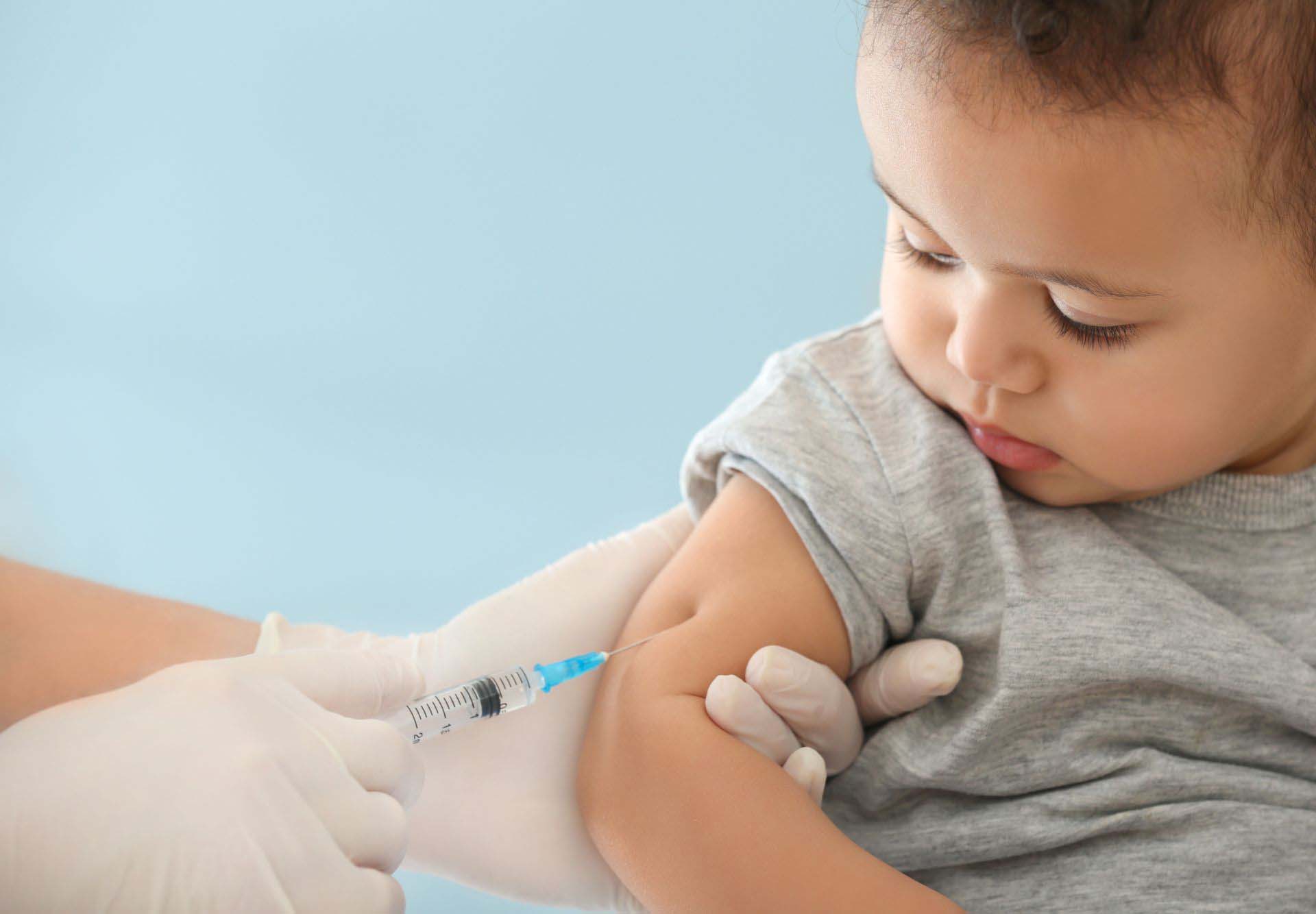
Pfizer has delayed submitting its request for FDA approval of the COVID-19 vaccine for children under 5 years old. The company made this decision to gather additional clinical trial data for a three-dose regimen instead of the originally proposed two-dose schedule. Pfizer is confident that a third dose will elicit a stronger immune response in young children based on the results observed in trials with two doses.
Parents eagerly awaiting the opportunity to vaccinate their young children will be disappointed by this update. The Pfizer COVID-19 vaccine received approval in May 2021 for adolescents aged 12 to 15, followed by approval in late October 2021 for children aged 5 to 11.
During the Omicron surge, there was a notable increase in hospitalizations of young unvaccinated children. Parents of infants, toddlers, and preschoolers waited anxiously for confirmation that their children would be safeguarded from the severe effects of COVID-19.
Exploration of Pfizer’s COVID-19 Vaccine in Young Children
Following a surge in Omicron infections in early February, the Food and Drug Administration (FDA) requested trial data from Pfizer for Emergency Use Authorization (EUA) of the vaccine in young children. While awaiting the trial results, children aged 2 to 4 could potentially receive the two-dose vaccine, with the option of a third dose for enhanced protection.
The progress of Pfizer’s three-dose trial exceeded expectations, prompting a revision of the initial strategy. Pfizer was confident in the expedited collection of trial data, anticipating a more robust immune response with the additional dose.
Pfizer and BioNTech maintain their belief in the potential of the three-dose regimen to offer superior protection in this age group. However, they have chosen to await the completion of the research before making any decisions.
The adjustment in strategy is not linked to any deficiencies in the vaccine but rather focuses on optimizing its effectiveness. Infants aged 6–24 months and children aged 2–4 were the primary participants in the initial immunization studies. These age groups received two doses of 3 µg each, spaced 21 days apart.
In comparison to the dosages administered to older children (10 µg for ages 5-11 and 30 µg for ages 12-15), the dosage for younger children was notably lower. No adverse effects were observed in the 6- to 24-month-old and 2- to 4-year-old groups after receiving the 3 µg dose.
Pfizer reports that infants who received the 3 µg dosage exhibited an immune response similar to that of healthy adults aged 16–25 after vaccination. However, children aged 2–4 displayed a weaker immune response, prompting Pfizer to investigate the potential benefits of a third 3 µg dose for this age group. Pfizer anticipates swift data collection, with results from the three-dose trial expected by early April 2022.
The temporary halt in the trial was due to the insufficient antibody protection observed with the lower vaccine dose in this age category. Pfizer’s primary concern is providing a safe vaccine that offers children the same level of protection as other age groups.
Significance of the COVID-19 Vaccine for Young Children
Despite Pfizer’s decision to postpone the vaccine application, experts emphasize the critical need for a vaccine for younger children. While most children recover quickly from COVID-19, unvaccinated children are particularly vulnerable to severe complications, as evidenced during the Omicron surge in New York.
Pediatric hospitalizations due to COVID-19 surged in New York City, with the majority of hospitalized children being unvaccinated. A significant portion of these children were under the age of four, an age group previously ineligible for vaccination.
The availability of a vaccine that protects young children from the virus will offer parents peace of mind regarding their children’s well-being. Previously, such reassurances were only extended to children aged 5 and above.
Approval of this vaccine would enable entire households to be vaccinated against COVID-19, with the exception of infants under six months old. The spike in infections among children during the Omicron wave led to disruptions in daycare and preschool operations, along with quarantine measures, posing challenges for both parents and children.
Vaccines play a crucial role in safeguarding children’s health and enabling them to resume normal activities like daycare, preschool, classes, outings, and celebrations, thereby mitigating the impact of COVID-19 on their lives and families.
While parents may feel disheartened by the delay in vaccine availability for young children, it is essential to recognize that the vaccine is expected to be accessible in the near future. In the meantime, implementing safety measures like social distancing and mask-wearing can help protect young children.
For more insightful articles, consider exploring the following recommendations: Your Family And The Covid-19 Vaccine, Comprehensive Information Regarding the Moderna Vaccine, Is Covid-19 Vaccine Only Available Via Shot?